CLIMATE AND WEATHER: When you see how difficult it is to get even next week’s weather forecast right, you can imagine the challenges researchers are faced with when it comes to predicting the weather decades from now.
Climatologists need good models to predict climate change over time, but to predict how the Earth’s climate is changing, they also need to understand how water droplets behave.
Lars Onsager
- Lived from 1903 to 1976
- Nobel Laureate in Chemistry, 1968
- Engineer from NTH, one of NTNU's predecessors. Affiliated with Johns Hopkins, Brown and Yale Universities
- You can read more about Onsager at the Nobel Prize pages
“Now, we are capable of describing the transfer of heat and mass across both planar (flat) and curved water interfaces,” says Øivind Wilhelmsen, a research scientist at SINTEF Energy Research.
Wilhelmsen’s research relates to nonequilibrium thermodynamics, and deals with an extension of the theories of the Norwegian Nobel Prize winner Lars Onsager. [See fact box] Most of us will find his research quite difficult to understand. However, it is of fundamental importance, not just for predicting tomorrow’s weather, but also for understanding how weather and climate are going to change in the years to come.
It turns out that the water cycle and precipitation are among the largest uncertainties in current climate models.
- You might also like:Big discoveries about teeny tiny particles
How water droplets grow
“Water evaporates all the time from oceans, rivers and lakes. Then, clouds form in the atmosphere. Tiny droplets form in the clouds and eventually fall down as rain when they have grown large enough. How quickly these processes occur, how large the clouds become and when the rain falls all depends on how fast mass and energy are transported across water interfaces,” says Wilhelmsen.

Very small water droplets form in clouds. Once they have grown large enough, the drops will eventually fall to Earth as rain. Photo: Thinkstock
Some of the uncertainty in current weather forecasts and climate models lies in our fundamental lack of understanding of these transport processes.
“How water droplets grow depends on their interface transfer coefficients, which Wilhelmsen has calculated,” says Professor Signe Kjelstrup from NTNU’s Department of Chemistry.
The research was part of Wilhelmsen’s doctoral thesis, for which Kjelstrup was a supervisor, along with Professor Dick Bedeaux.
Research with a wide range of applications
Many scientific fields can benefit from the research.
“This research is very general, and the results allow us to describe a wide range of processes across many scales, from evaporation from large lakes to the growth of water droplets that are only a few nanometres in size,” says Bedeaux.
Steam turbines
- Steam turbines generate approximately 80% of all electricity in the world.
- Power plants use coal, natural gas, or other energy sources to heat and vaporize water.
- The steam then passes through a steam turbine that drives an electric generator and creates our electricity.
- Evaporation and condensation of water are essential in this process.
Scientists can use the results to better understand natural processes, through weather forecasts and climate models. However, the findings also have industrial relevance, and are useful in industrial processes that involve evaporation or condensation of water. One important example is steam turbines, which are the most widely used equipment to generate electricity on a worldwide basis. [See fact box]
“For many years, this has been a missing piece of the puzzle for several important processes, both in nature and in industry,” explains Wilhelmsen.
“The findings are useful in a large number of applications, and we would like to see the results put to use,” says Kjelstrup.
Started with little
The researchers began with only scattered fragments of a description of how droplets behave. Their first task was to connect the right pieces. There was no satisfactory description even for completely flat water interfaces.
“Now, we are even able to describe curved interfaces,” Kjelstrup says.
Water is special, and its peculiar properties are one of the reasons why life was able to evolve on Earth. These same properties posed huge challenges, however.
“We had to use all the tools at our disposal—experiments at low temperatures, molecular dynamics simulations at high temperatures, and advanced new theory to make everything fit together. This would not have been possible ten years ago,” says Wilhelmsen.
Part of the challenge lies in fact that the relevant experiments can only be conducted at low temperatures. At high temperatures, researchers were able to use nonequilibrium molecular dynamics simulations, where they could mimic on a computer how real water molecules interact with each other in a simulated volume. These simulations allow researchers to capture the anomalous properties of water quite precisely. At lower temperatures however, the simulations became so computationally demanding, that they were impossible to carry out, even on the most powerful supercomputers available today. The scientists then had to use advanced theory to pull the pieces together.
The story continues under Figure 1.
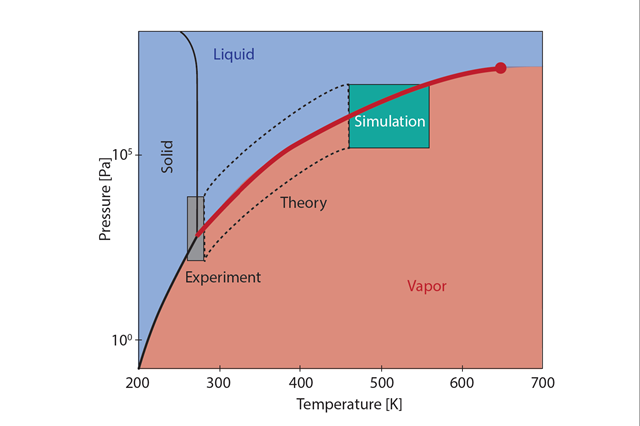
Figure 1: The water phase diagram, where the lines represent phase transitions where water changes from liquid to ice, from ice to vapor, or from vapor to liquid. For evaporation and condensation of water, we are interested in the phase transition between liquid and vapor, as illustrated by the thick red line. The red circle represents the critical point where we can no longer distinguish between vapor and liquid. At low temperatures, one can study the interface transfer coefficients of water with experiments; at high temperatures, one can use nonequilibrium molecular dynamics simulations and in the area between these, one must use advanced theory (in the box with dashed lines). Illustration: Øivind Wilhelmsen with help from Astrid B. Lundquist at SINTEF Energy Research.
How the curvature of water droplets influences weather and climate
When water droplets first form in the atmosphere, they are very small. They then grow almost a million times in size before they eventually fall as raindrops. If we can determine exactly how fast water droplets grow under certain conditions, we can more easily predict when and how much it is going to rain.
“Since the water droplets are very small when they first form, the curvature of their interfaces affects how fast they grow,” says Wilhelmsen.
The water droplets first grow by absorbing water from the atmosphere, and they are almost spherical in shape during this process. When they have become big enough, two water droplets can also collide and merge, or coalesce, into a bigger drop.
“We are now, for the first time, capable of describing how the transport of heat and mass occurs across water interfaces as two water droplets are coalescing. Here, the geometry and the curvature become more complicated,” says Professor Bedeaux.
The figure below shows what the thermal resistance of the interface looks like when two nanosized water droplets coalesce to become a bigger drop. Here, the blue color represents high resistance. The figure shows that it is difficult to transfer mass and heat into or out of the region where the two droplets are merging.
“Water droplet coalescence is an important mechanism for precipitation of rain in the tropics,” says Wilhelmsen.
The story continues under Figure 2.
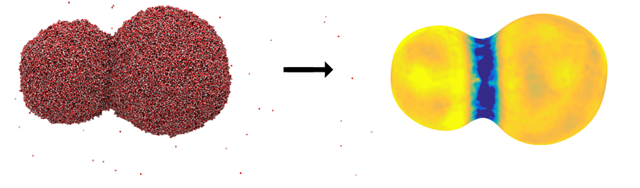
Figure 2: An image of two coalescing water droplets. The figure to the left shows a snapshot from a molecular dynamics simulation, where the small dots represent water molecules. The figure to the right illustrates the interface transfer coefficient for heat transfer, where the blue color means that the resistance to transfer is large. It is difficult to transport heat into or out of the region where the two droplets merge. Illustration:Øivind Wilhelmsen, SINTEF Energy Research
Fundamental research
The scientists want to emphasize that their work is fundamental research that provides a new understanding of processes that we have known about for a long time. But at the same time, the consequences of the findings can be large.
“We have now figured out a new piece of the puzzle. This piece can be used in climate models and weather forecasts to improve our understanding—not only of how the weather will be like tomorrow, but also of how weather and climate will evolve in the future. It is important to reduce the uncertainty in current climate models because this will allow us to convince more people that it is super-important to act as quickly as possible to do something about the global warming,” says Wilhelmsen.
The research has been published in Physical Review E and was undertaken in collaboration with the Institute of Fluid Mechanics at the Friedrich-Alexander University Erlangen-Nuremberg in Germany. Wilhemsen’s co-authors are D Thuat T. Trinh, Anders Lervik and Vijay Kumar Badam, along with Kjestrup and Bedeaux.
You can read more about the article here: Coherent description of transport across the water interface: From nanodroplets to climate models. Øivind Wilhelmsen, Thuat T. Trinh, Anders Lervik, Vijay Kumar Badam, Signe Kjelstrup, and Dick Bedeaux. Phys. Rev. E 93, 032801

