The green transition is dependent on the availability of sufficient quantities of critical raw materials, such as the metals manganese and silicon, which are used in batteries, electric vehicles, wind turbines, and more. In nature, manganese and silicon cannot be found in metallic form; they are bound to oxygen in various minerals.
To extract the metals, they must be separated from the oxygen, typically using fossil carbon. At high temperatures, the carbon reacts with the oxygen, leaving behind pure metal. Unfortunately, the product of a reaction between oxygen and carbon is the greenhouse gas CO2, which becomes an unavoidable by-product in many metal-producing processes. Take for example the simplified reaction equation for the production of silicon (chemical formula Si):
SiO2+C=Si+CO2
Since carbon is fundamentally linked to the metal production processes, decarbonizing electricity production alone will not be sufficient. Even with 100% renewable electricity, the production of manganese, silicon, and many other metals will still result in CO2 emissions with current production technology. Hydrogen (H2) is seen by many as a solution, as it can replace fossil carbon in the production of many types of metals. When hydrogen reacts with oxygen, water vapor is formed, eliminating direct CO2 emissions. For example, many are working on such solutions for the production of iron (chemical formula Fe):
FeO+H2=Fe+H2O
Unfortunately, nature does not cooperate when it comes to silicon and manganese. These metals are so strongly bound to oxygen that they will not react with hydrogen.
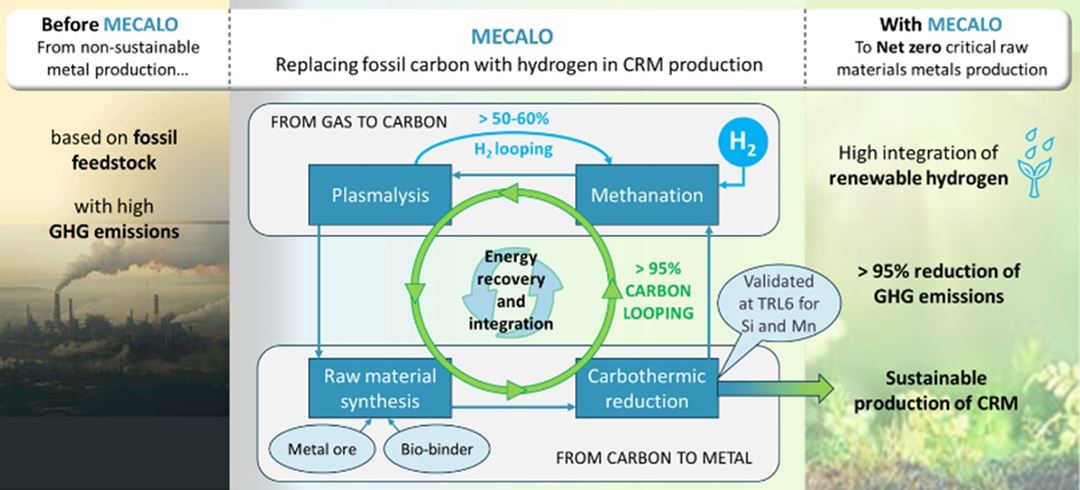
MeCaLo stands for Metal production with Carbon Looping, where the idea is that instead of storing CO2, it will be converted back into a solid carbon raw material that can replace fossil carbon in metal production. Renewable hydrogen is used for this through a two-step process. The first step is to convert CO2 into the gas methane, CH4, which is also the main component of natural gas. This process is known as methanation and is done using a catalyst:
CO2+4H2=CH4+2H2O
In the next step, energy in the form of electricity is added, and methane is decomposed into hydrogen and solid carbon (C):
CH4+energi=C+2H2
The resulting carbon, a fine powder, must then be processed and made suitable for use as a raw material in a smelting furnace.
SINTEF's contribution involves developing new catalysts for the first step in the conversion of CO2, i.e., methanation. We will also conduct extensive testing of the produced carbon raw material to ensure it can be used in metal production. Additionally, we will demonstrate scalability by producing several hundred kilograms of manganese in our pilot-scale smelting furnace.