
Research group of Process Chemistry and Functional Materials
Electrification
Examples of such processes are: electrochemical production of chemicals, fuels and materials (power-to-products) and innovative use of electricity such as plasma, microwaves and ultrasound to power processes.
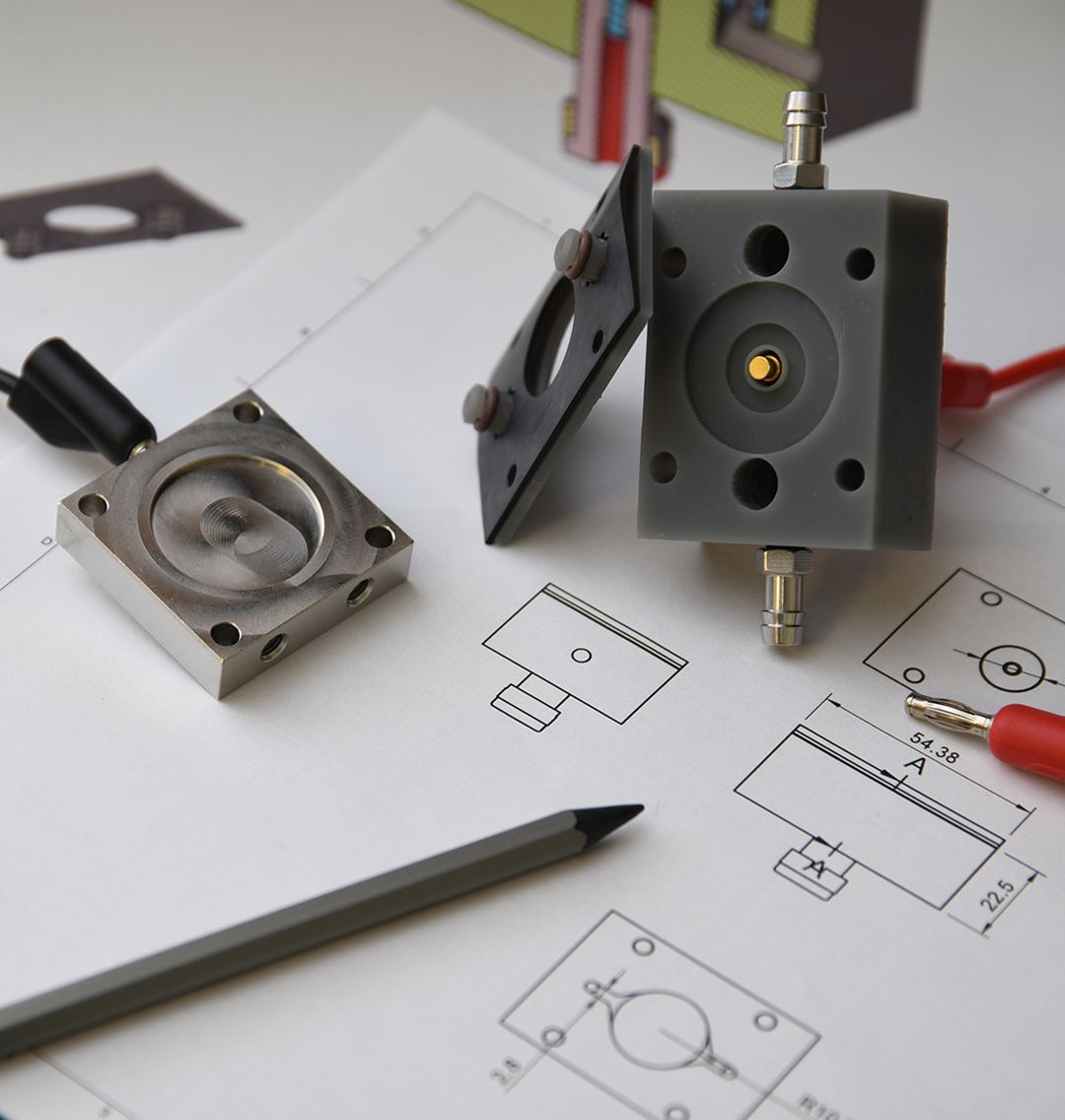
We use electrical potential to drive redox reactions in liquid and gas phase. The benefits are rapid changes in oxidation/reduction potential, immediate and highly sensitive feedback on conversion speed, and good reaction control. We work with both development of electrode materials, cell/reactor design, testing and characterization, supported by atomic scale and process modeling.
Our test systems operate in both flow and batch modes and are, among other things, suitable for organic and inorganic synthesis, removal of harmful compounds, and conversion of waste and by-products into more valuable components.
Projects:
- EBIO – Biofuels Through Electrochemical Transformation Of Intermediate Bio-Liquids
- HYPER – An electrochemically produced oxidiser for modular, onsite generation of HYdrogen PERoxide
- NoViCo - Novel biorefinery concepts for valorization of lignocellulosic residues
- NCS C+ – The Norwegian Continental Shelf: A Driver for Climate-Positive Norway
- PhotoRed – Photocatalytic and photoelectrochemical carbon dioxide reduction
- Synergistic metal/non-metal doping of titanium dioxide to produce hydrogen under UV and Vis light
- WoBiCo – From Wood to Sustainable Biocomposites